
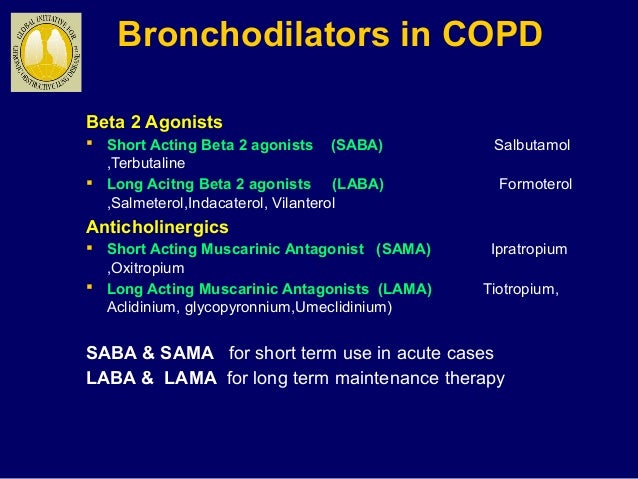
Recently, a reduction of the mortality has also been shown by this treatment. If the patients have severe symptoms and a history of exacerbations, the addition of inhaled corticosteroid (ICS) to long-acting muscarinic antagonist (LAMA) and long-acting beta-agonist (LABA) combination therapy has been recommended because it lowers the incidence of exacerbations. To reduce the symptoms and the exacerbation, single or dual inhaled bronchodilators are recommended for the treatment depending on the severity. The symptoms include dyspnea, cough and sputum production and worsen during exacerbations of COPD, which are associated with accelerated mortality.
#LAMA LABA COPD TRIAL#
However, these results should be only applied to patients with symptomatic moderate to severe COPD and a history of exacerbations.Ĭlinical Trial Registration: PROSPERO CRD42020191978.Ĭhronic obstructive pulmonary disease (COPD) is the third leading cause of death in the world. In addition, triple therapy is also superior to LABA/LAMA due to the lower mortality and better dyspnea score. Triple therapy causes a higher incidence of pneumonia but is a more preferable treatment than LAMA/LABA due to the lower incidence of exacerbations, higher trough FEV 1 and better QOL score. Concerning the trough FEV 1, QOL score and dyspnea score in both protocols, the differences were less than the minimal clinically important difference. In the ICS-withdrawal protocol including 2 RCTs, triple therapy also showed a significantly better QOL score and higher trough FEV 1 than LAMA/LABA. However, triple therapy showed a significantly higher incidence of pneumonia (odds ratio 1.52, 95% CI 1.16–2.00). In addition, triple therapy significantly improved the dyspnea score (mean difference 0.33, 95% CI 0.18–0.48) and mortality (odds ratio 0.66, 95% CI 0.50–0.87). ICS/LAMA/LABA treatment (triple therapy) significantly decreased the incidence of exacerbations (rate ratio 0.73, 95% CI 0.64–0.83) and improved the QOL score and trough FEV 1 compared to LAMA/LABA. We identified a total of 6 RCTs in ICS add-on protocol (N = 13,579). We searched relevant randomized control trials (RCTs) and analyzed the exacerbations, quality of life (QOL), dyspnea symptom, lung function and adverse events including pneumonia and mortality, as the outcomes of interest. Therefore, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety including ETHOS trial. Recently, a large new ETHOS trial has been performed to clarify the ICS add-on effects. However, the evidence is mainly based on one large randomized controlled trial IMPACT study, and it remains unclear whether the ICS add-on treatment is beneficial or not. In addition, a reducing effect on mortality has been shown by this treatment. Recently, the addition of inhaled corticosteroid (ICS) to long-acting muscarinic antagonist (LAMA) and long-acting beta-agonist (LABA) combination therapy has been recommended for patients with COPD who have severe symptoms and a history of exacerbations because it reduces the exacerbations.


 0 kommentar(er)
0 kommentar(er)
